Background of Fluorescence and Stereology Integration
By Darren Tran and Peter R. Mouton.
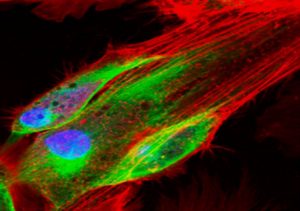
Immunofluorescent staining offers a range of benefits over conventional immunostaining, including: higher signal: noise (S:N); superior spatial resolution; specific co-labeling of multiple objects in the same sections; and direct correlation between fluorescence intensity and antigen concentration in tissue sections.
During Customer Discovery interviews in preparation for deployment of our AI-based Fully Automatic Stereology Technology (FAST®), a significant number of current and future SRC customers evinced an interest in expanding this NSF-funded approach to automatic quantification of fluorescent images. To address this unmet need, SRC Biosciences has entered into collaboration with industry partners, i.e., global providers of microscopes for wide-field deconvolution and confocal imaging to develop FluoroStereologer, the first approach that combines deep learning with the optical fractionator and other unbiased stereology methods.
This expansion of AI to immunofluorescent-stained cells and subcellular structures will establish a new state-of-the-art approach for stereology quantification of Number, Volume, Surface Area, Length and Variation that covers the full spectrum of low-to-high resolutions possible with fluorescent imaging.
Figure 1: Fluorescence microscopy performed to visualize an aggregation of cells.
How does FluoroStereologer work?
Image Capture (SRC). In step 1, our experts will outline an anatomically defined region in 8 to 12 sections containing high signal: noise (S:N) images of immunofluorescent-stained biological targets, e.g., fluorescent cells, endosomes, mitochondria. In step 2, the software will drive the microscope’s motorized XYZ stage to capture ~ 100 to 150 images or images stacks, e.g., disector stacks inside the outlined region. These images will be captured at cellular (40x) or subcellular (60-100x) resolutions, depending on needs of the project.
AI-based Deep learning (SRC). In step 3, our histology experts will generate a set of “ground truth” images by annotating (clinking an “x”) on the targets of interest, thereby generating a “training set” for deep learning. In step 4, our software development team will use these annotated images to train a “model” that will learn to automatically recognize the annotated targets. In the final step, we will validate the model by comparing the accuracy of the deep learning data with that collected from the same images using manual stereology. Minimum performance standards relative to manual stereology of the same images are high accuracy (< 5% error); 100% reproducibility (Test/Retest); and high efficiency (10-20x faster).
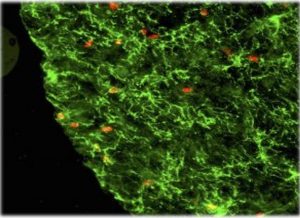
Figure 2: Fluorescence microscopy performed to visualize objects of interest in a ball cluster.
How will FluoroStereologer work for end-users:
The end-user will use FluoroStereologer in a two-step process. First, the user will capture similarly immunofluorescent-stained images from an anatomically defined region. Second, the validated model will quantify the targets in one of two modes: semi-automatic (with user approval) or fully automatic (without user approval). The FluoroStereologer will allow the end-user to check accuracy on a sub-sample or all images by manual stereology on the same study images. The final results will provide study results, e.g., total cell number by automatic and manual methods, as well as coefficient of error (CE) for all stereology estimates and descriptive statistics (mean, SD, SEM) for the study.
For more information, see our blog post on fluorescence, titled: Seeing with Colors.
Reference: Mouton, P.R. (2002) Principles and Practices of Unbiased Stereology: An Introduction For Bioscientists. The Johns Hopkins University Press, Baltimore, MD.