By Darren Tran and Peter R. Mouton – Alzheimer’s, Parkinson’s and other neurodegenerative diseases are a large and growing cause of morbidity and mortality in elderly populations. Among the major findings in afflicted humans and analogous animal models is a strong association between progressive cognitive decline and dendrite degeneration in the neocortex and hippocampus.
Golgi-Cox staining with stereological quantification of dendritic length provides a powerful approach for quantifying this association in behaviorally tested rodent models. In drug discovery studies, this study design allows for preclinical testing of potentially novel strategies for the therapeutic management of afflicted patients.
The Golgi-Cox Staining Method for dendrite analysis
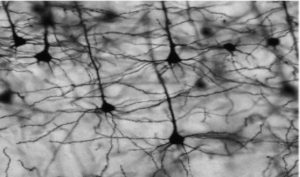
Although the exact details of the staining mechanism remain unclear [1], Golgi-Cox staining uses mercuric chloride for complete impregnation of a small fraction of neurons in neocortical and hippocampal regions [Figure 1]. Importantly, the technique stains neurons in their entirety, allowing for clear quantification of cell soma, axons, dendrites, and spines under medium to high microscopic magnification. The effectiveness of Golgi-Cox integrates well with fluorescence and visible light immunocytochemistry as an overall approach for assessing neuropathology, toxicology, etc. Furthermore, the low background of Golgi-Cox staining method provides strong relief to dendritic branches as seen in Figure 1.
Figure 1. Neurons in the hippocampus region that are even stained through the Golgi-Cox method. [1]
To minimize data inconsistencies, the Golgi-Cox staining method follows careful preparation protocols for preparation of the staining solutions, transferring the brain tissue and tissue sectioning. Several quality control steps help to maximize results such cutting the brain along the sagittal plane to preserve the dendritic branches, using a tissue protectant to preserve tissue slices, reducing staining background and increasing adherence of tissue sections to the microscope slide [2]. Finally, Contract research organizations experienced with stereology studies allow for more accurate data collection.
Applications of stereology in Golgi spine analysis
Cell quantification in each tissue section is examined using computerized stereology by the Stereologer system. Stereology introduces a systematic random sampling methodology that avoids bias in selecting neurons that demonstrate complete Golgi impregnation from the Golgi-Cox staining.
A sampling step length is specified to allow for a sample size large enough for Golgi spine analysis (different based on the study). A magnification is set to characterize the optical disector that has strict inclusion criteria. If a neuronal cell body falls within the optical disector, the neuron would be accepted and analyzed. If it falls outside the optical disector or the cell body hits the exclusion line, the cell is not analyzed [3]
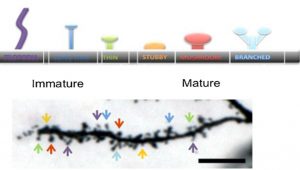
The stereological parameters most often analyzed are cell diameter, number of dendritic branches, dendrite length, number of spines, and spine maturity as characterized by the spine shape. The goal is to quantify dendritic degradation, a consistent finding in diverse neurodegenerative diseases. As shown Figure 2, the number of spines is counted over the length of the dendrite obtained and the spine shape characterizes the spine maturity [2]
Figure 2. Illustration of how spine shape over the length of the dendrite characterizes overall maturity of the dendrite. [2]
In conclusion, the integration of the Golgi-Cox staining method and stereology offers accurate and unbiased methods to study neuronal bodies. Stereology is also applicable to other staining methods such as fluorescence and immunostaining. Finally, as many immunohistochemistry labs typically have a microscope with a camera and motorized stage, our state-of-the-art stereology software can be easily implemented to benefit your research.
Cited Works and Additional Reading:
[1] Zaqout, S., & Kaindl, A. M. (2016). Golgi-Cox Staining Step by Step. Frontiers in neuroanatomy, 10, 38. https://doi.org/10.3389/fnana.2016.00038
[2] Bayram-Weston, Z., Olsen, E., Harrison, D. J., Dunnett, S. B., & Brooks, S. P. (2016). Optimising Golgi-Cox staining for use with perfusion-fixed brain tissue validated in the zQ175 mouse model of Huntington’s disease. Journal of neuroscience methods, 265, 81–88. https://doi.org/10.1016/j.jneumeth.2015.09.033
[3] Mouton, P. R. (2002). Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists (1st ed.). Johns Hopkins University Press.